Back to basics

All vehicles need a battery to start and operate, but do we understand what is happening inside that black plastic box? The following information steps through it from an electrical and chemical perspective.
A battery is an electrochemical device which is used to accumulate and store chemical energy which can be released as electrical energy upon demand. There are a number of different types of lead acid batteries (i.e. Calcium, EFB & AGM), however they all use the same electrochemical reaction to store and deliver power.
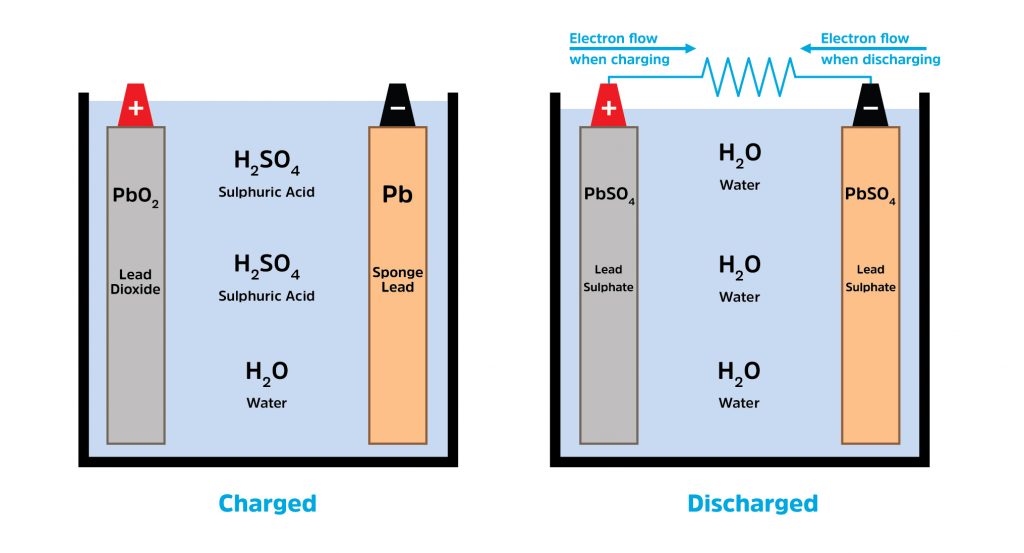
When a lead acid battery is fully charged, there is an excess of electrons on the negative plates (electrons are negatively charged). The positive plates do not have any electrons, which creates a high potential difference (i.e. voltage) between the positive and negative plates.
When an electrical device is connected, electrons begin to flow (i.e. current flow) from the negative side along the conductor and through the device to the positive side. As more electrons flow to the positive plate, the voltage is reduced and the battery discharges. This movement of electrons through the device is what makes it operate (e.g. creates movement, heat, light etc.).
A battery charger reverses this process by forcing electrons back to the negative plates. When there is a movement of electrons, the battery undergoes a chemical change which enables the battery to store energy.
A fully charged lead acid battery is made up of three main elements. The active material on the positive plates is lead dioxide (PbO2), and the negative plate active material is called sponge lead (Pb). The electrolyte is a diluted solution of sulfuric acid (H2SO4 and H2O).
As a battery discharges, the electron flow allows the oxygen (O24-) molecules to be released from the lead dioxide at the positive plates. Oxygen has a higher attraction to hydrogen (H+) than the sulphate molecule, so hydrogen breaks its bond with the sulphate and combines with oxygen to create water (H2O). The now free sulphate molecules (SO42-) combine with the available lead molecules (Pb2+) at both plates creating lead sulphate (PbSO4).
Although there are new lightweight and more compact battery technologies available (e.g. lithium), almost all new vehicles still being fitted with lead acid batteries. Why? The lead acid battery is a mature technology which even now is still being developed and improved. It is low cost, safe and highly recyclable – more than 96% of a lead acid battery can be recycled.